- Global Logistics
- EXIM Certifications
- Intelligence
- Technology
- Resource
- Company
-
Global Logistics
-
EXIM Certifications
-
Intelligence
-
Technology
-
Resource
-
Company
Refer to teammate
We'll never share your email with
anyone else.
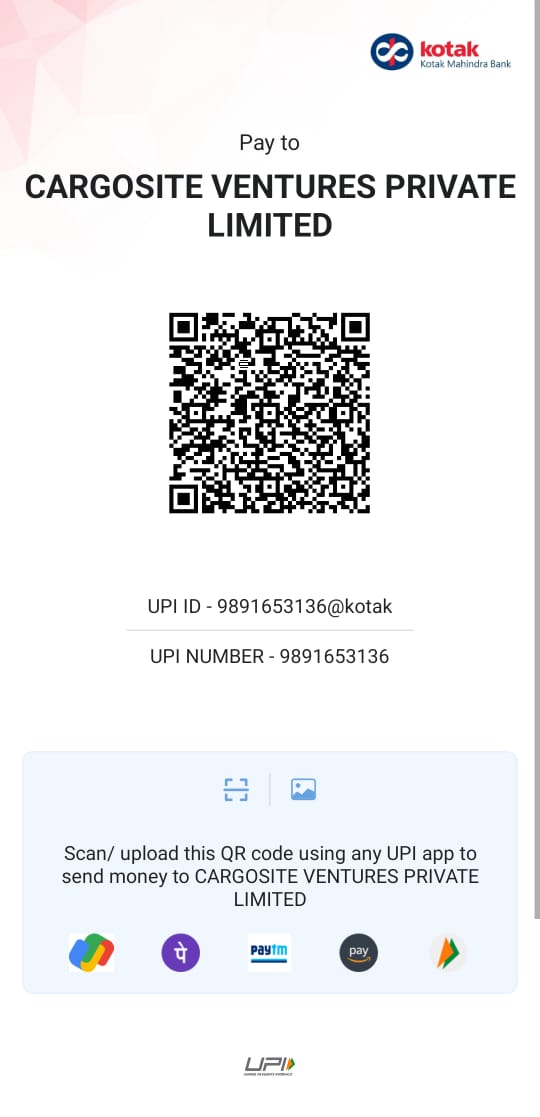
Pay By QR